Researchers report collaborative regulation of yeast sphingolipid synthesis by phosphorylation and ceramide
DATE:2024-02-06
In eukaryotes, serine palmitoyltransferase (SPT) enzymes catalyze the first and rate-limiting step in the de novo synthesis of sphingolipids. Orm family proteins are negative regulators of SPT and regulate SPT activity by directly binding to SPT. Yeast Orm has a phosphorylation regulatory mechanism that is absent in higher eukaryotic organisms. When the sphingolipid level in cells is low, Ypk1 kinase can phosphorylate the N-terminus of Orm protein, thereby releasing Orm’s inhibitory effect on SPT.
In yeast, SPT and Orm can form a stable complex with phosphoinositide phosphatase Sac1, called the “SPOTS” complex. Although the previous studies from Professor Xin Gong’s research group from the School of Life Science at the Southern University of Science and Technology (SUSTech) have indicated a conserved ceramide-mediated feedback inhibition of SPT-ORM/ORMDL complexes in higher eukaryotes, its conservation and relationship with phosphorylation regulation in yeast remain unclear.
Professor Xin Gong’s group recently published a study, in collaboration with Professor Teresa M. Dunn’s research group at the Uniformed Services University of the Health Sciences (USU) in the USA to reveal that the yeast SPT-Orm2 complex maintains sphingolipid homeostasis through the mechanism of collaborative regulation by phosphorylation and ceramide.
Their research paper, entitled “Collaborative regulation of yeast SPT-Orm2 complex by phosphorylation and ceramide”, has been published in Cell Reports, a journal covering a broad range of disciplines within the life sciences.
The researchers obtained the yeast SPOTS complex through in vitro recombinant expression and purification. To study the molecular mechanism of sphingolipid homeostatic regulation by the SPOTS complex under the dephosphorylated state of Orm2, they conducted cryo-electron microscopy (cryo-EM) analysis of the SPOTS complex in a dephosphomimetic state.
Through the optimization of protein samples, the high-resolution cryo-EM structure of the SPT-Orm2 complex, except for the Sac1 subunit, was obtained. Analysis of the structure revealed that ceramide binds and stabilizes Orm2 in an inhibitory conformation that inhibits SPT activity. Subsequently, the researchers generated a phosphomimetic mutant of Orm2. Combining structural and biochemical experiments, it shows that phosphorylation of Orm2 could break its inhibitory conformation, thereby releasing the inhibition of SPT activity.
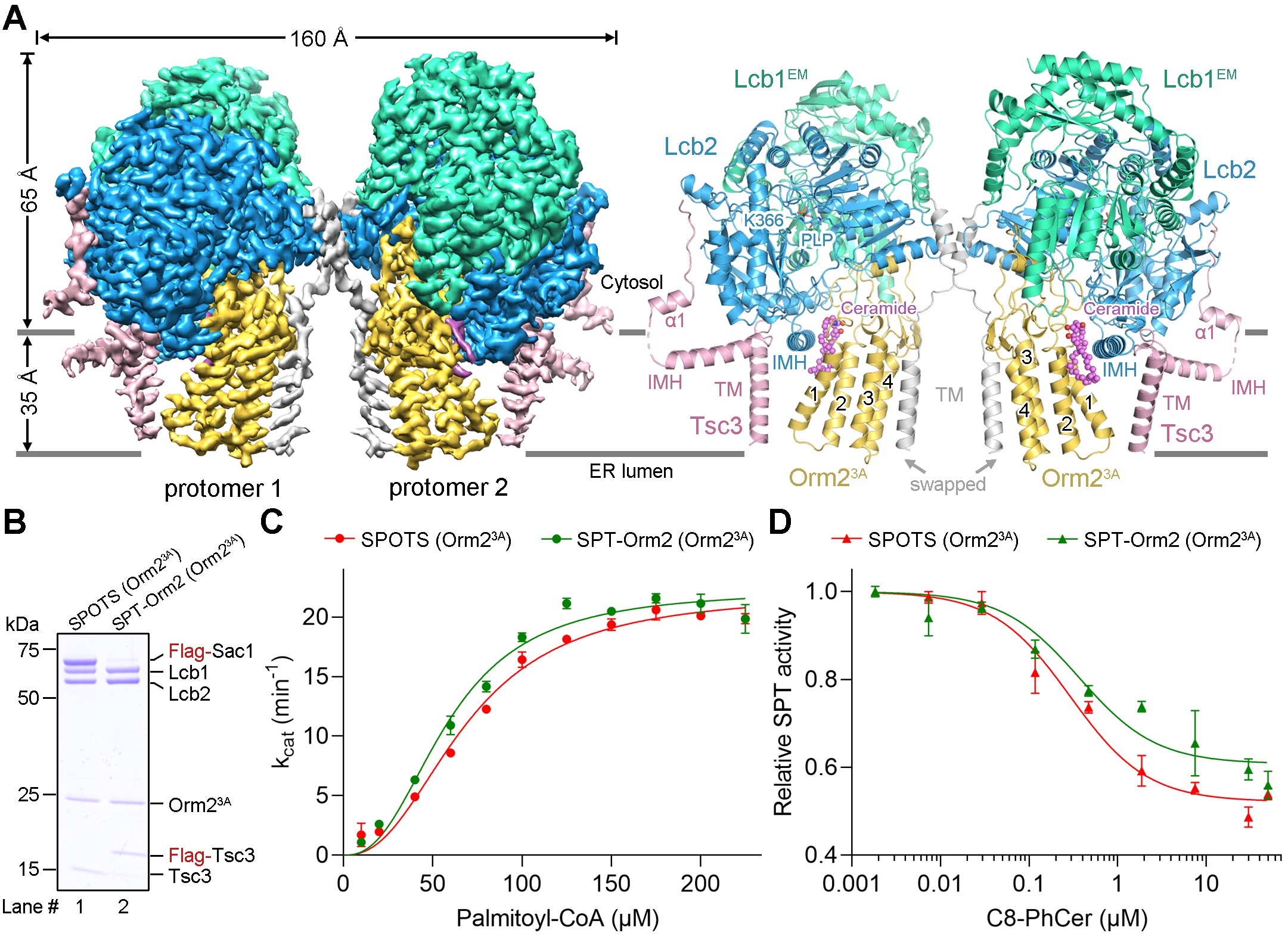
Figure 1. Overall structure of the yeast SPT-Orm2 complex
Based on the structural analysis, biochemical experiments, and in vivo results, the researchers proposed a working model for the collaborative regulation of SPT activity by phosphorylation and ceramide in yeast (Figure 2). In this model, under low sphingolipid levels, Ypk1 phosphorylates the N-terminus of the Orm2 protein, thereby maintaining the SPT-Orm2 complex in an ‘activated state’.
The phosphorylated SPT-Orm2 complex enhances sphingolipid biosynthesis to restore cellular sphingolipid levels. As cellular sphingolipid levels increase and reach high levels, two factors are required to turn the SPT-Orm2 complex into an ‘inhibited state’: dephosphorylation of Orm2 and ceramide binding. During the switching process between the ‘active state’ and the ‘inhibitory state’ of the SPT-Orm2 complex, there may be two intermediate states; one is where Orm2 remains phosphorylated, but the SPT-Orm2 complex is bound with ceramide; the other is where Orm2 is dephosphorylated, but the SPT-Orm2 complex lacks ceramide binding.
Taken together, this study suggests that both Orm dephosphorylation and ceramide binding are crucial for suppressing SPT activity in yeast. This highlights a distinctive regulatory mechanism in yeast involving the collaborative actions of phosphorylation and ceramide.
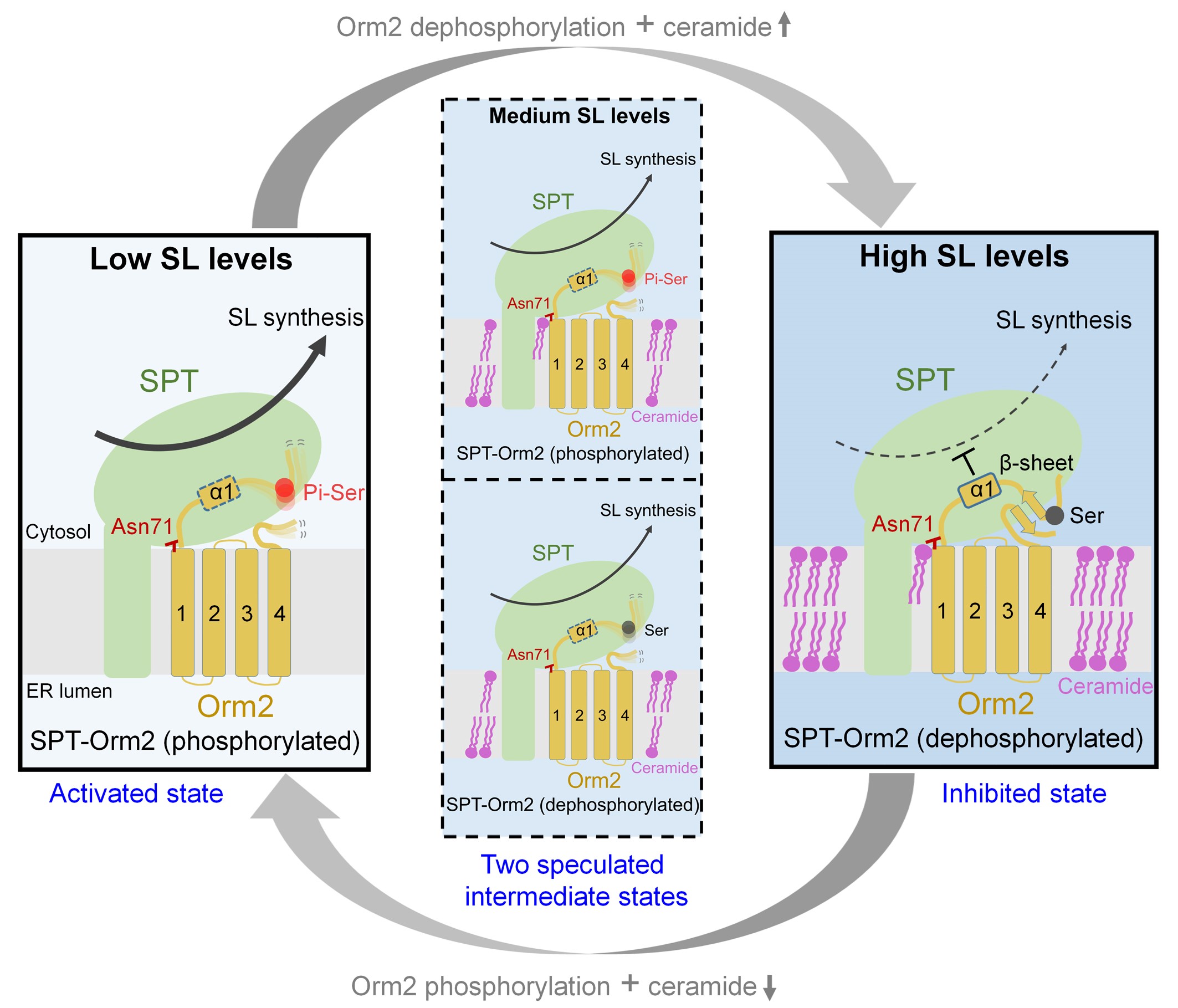
Figure 2. A working model for the collaborative regulation of yeast SPT-Orm2 by phosphorylation and ceramide
Research Associate Professor Tian Xie, doctoral student Feitong Dong, and master’s student Xinyue Wu, all members of Prof. Xin Gong’s research group, and Gongshe Han, a member of Professor Teresa M. Dunn’s research group at USU, are the co-first authors of this paper. Professors Xin Gong and Teresa M. Dunn are the co-corresponding authors.
This research was supported by the National Natural Science Foundation of China (NSFC) and the Shenzhen Science and Technology Innovation Commission. The cryo-EM data collection and processing for this work was supported by the Cryo-EM Center of SUSTech.
latest news
-
Dynamic changes in transposable elements shape human three-germ-layer differentiation
Date:2025-09-04
-
Researchers collaborate to uncover how SOD1 protects lysosome through autophagy
Date:2025-08-26
-
Researchers find 5-IP7 disrupts intestinal epithelial barrier and drives inflammation-induced colorectal cancer
Date:2025-08-26
-
Researchers decode molecular architecture and inhibition mechanism of human taurine transporter
Date:2025-08-22
