Researchers reveal the working mechanism of hATP13A2 transport polyamine
DATE:2023-04-09
Ubiquitously distributed polyamines are essential for many biological processes, including cell proliferation, differentiation, neuroprotection, and apoptosis. Polyamine homeostasis is essential for cellular viability and cell growth. Therefore, accurate regulation of polyamine homeostasis in human cells is necessary to maintain cellular fitness. As an essential compartment in the polyamine transport system, the lysosome plays a critical role in balancing polyamine concentrations between the cytosol and lysosome. The lysosomal transporter ATP13A2 was reported to export polyamines from the lysosome to the cytosol. ATP13A2 belongs to the P5B-type family, and substrate transport follows a process known as the Post-Albers reaction. Mutations of human ATP13A2 (hATP13A2) have been directly associated with the development of hereditary diseases like Kufor–Rakeb Syndrome (KRS; a type of juvenile-onset Parkinson's disease) and autosomal recessive spastic paraplegia 78 (SPG78).
Recently, Professor Zhong-min Liu's research group from the School of Life Science at the Southern University of Science and Technology (SUSTech), together with Professor Yong Wang's group from the College of Life Sciences of Zhengjiang University, revealed a full conformational cycle of human polyamine transporter ATP13A2.
Their research work, entitled "Conformational cycle of human polyamine transporter ATP13A2," has been published in Nature Communications, facilitating our understanding of the pathogenic mechanism of hATP13A2 in neuron diseases.
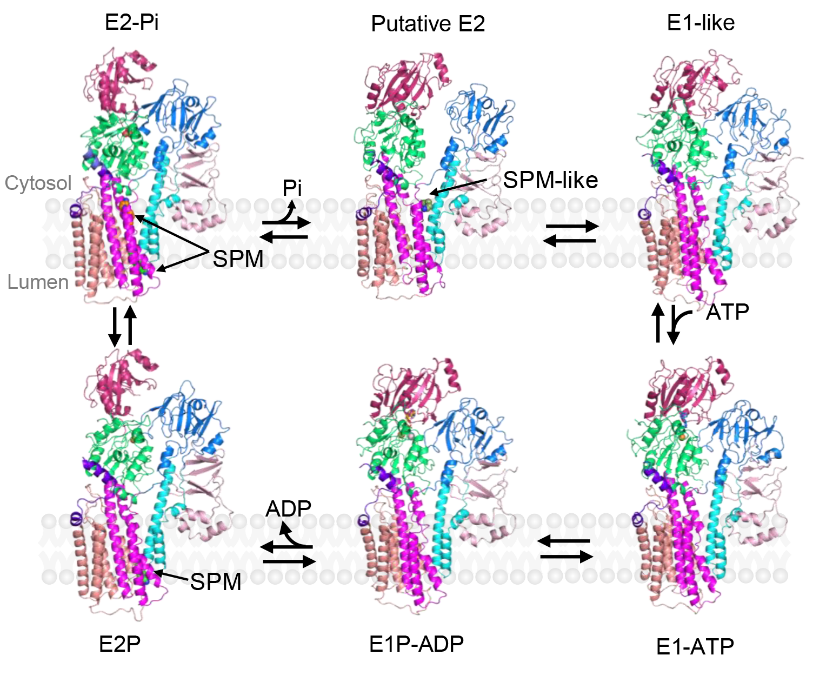
Figure 1 Atomic models of hATP13A2 in six intermediate states along the Post-Albers transporting cycle
This study reported seven cryo-EM structures of hATP13A2 in six intermediate states, providing a nearly complete atomic-level conformational cycle spanning the Post-Albers reaction process (Figure 1). Integration of high-resolution structures, biochemical assays, and molecular dynamics (MD) simulations revealed a new mechanism; unlike other P-type ATPases, hATP13A2 may transport substrates via a unique mechanism featuring an inward-opening cavity as a substrate buffering tank. The release of polyamine substrates by hATP13A2 is related to the affinity of the substrates to the binding sites and their local concentrations, which are promoted by phospholipids (Figure 2).
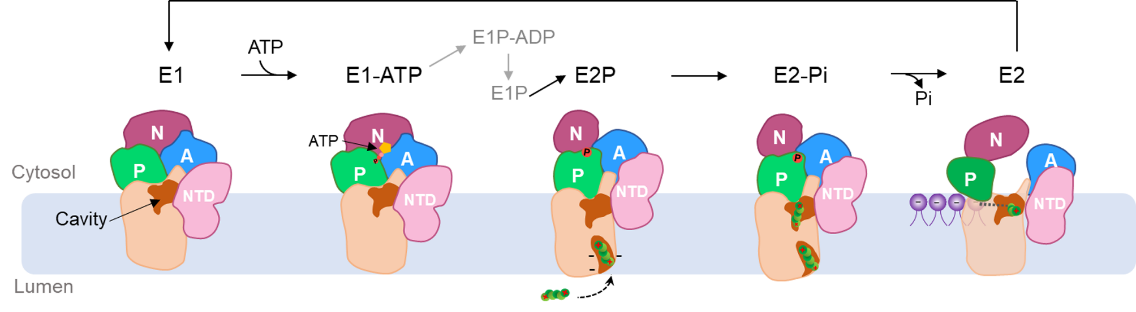
Figure 2 A proposed model of hATP13A2 transporting polyamines
Prof. Zhong-min Liu and Prof. Yong Wang are the co-corresponding authors. Graduate students Jian-qiang Mu and Chen-yang Xue from Prof. Liu’s research group, as well as Dr. Lei Fu from Zhengjia University, contributed equally to this study. This research was supported by the National Natural Science Foundation of China (NSFC) and Shenzhen Municipal Basic Research projects. Additionally, the cryo-electron microscopy data collection and processing was supported by the Cryo-Electron Microscopy Center of SUSTech and Shuimu BioSciences Ltd. The mass spectrometry experiments were supported by Prof. An-cheng Huang from the School of Life Science at the Southern University of Science and Technology, Prof. Hui-lin Li from the School of Pharmaceutical Science of Sun Yat-sen University, and the Mass Spectrometry System at the National Facility for Protein Science in Shanghai (NFPS).
Paper Link: https://doi.org/10.1038/s41467-023-37741-0
latest news
-
Dynamic changes in transposable elements shape human three-germ-layer differentiation
Date:2025-09-04
-
Researchers collaborate to uncover how SOD1 protects lysosome through autophagy
Date:2025-08-26
-
Researchers find 5-IP7 disrupts intestinal epithelial barrier and drives inflammation-induced colorectal cancer
Date:2025-08-26
-
Researchers decode molecular architecture and inhibition mechanism of human taurine transporter
Date:2025-08-22
