Revealing proteolytic activation for Hedgehog morphogen release
DATE:2021-12-01
The Hedgehog (Hh) signaling pathway is involved in orchestrating embryonic development and tissue homeostasis. Its dysregulation leads to various human diseases, including cancer and congenital malformations. The pathway is activated by the secreted Hh ligand, synthesized by its C-terminal intein domain (Hh-C) to generate an N-terminal environment (Hh-N) attached to cholesterol.
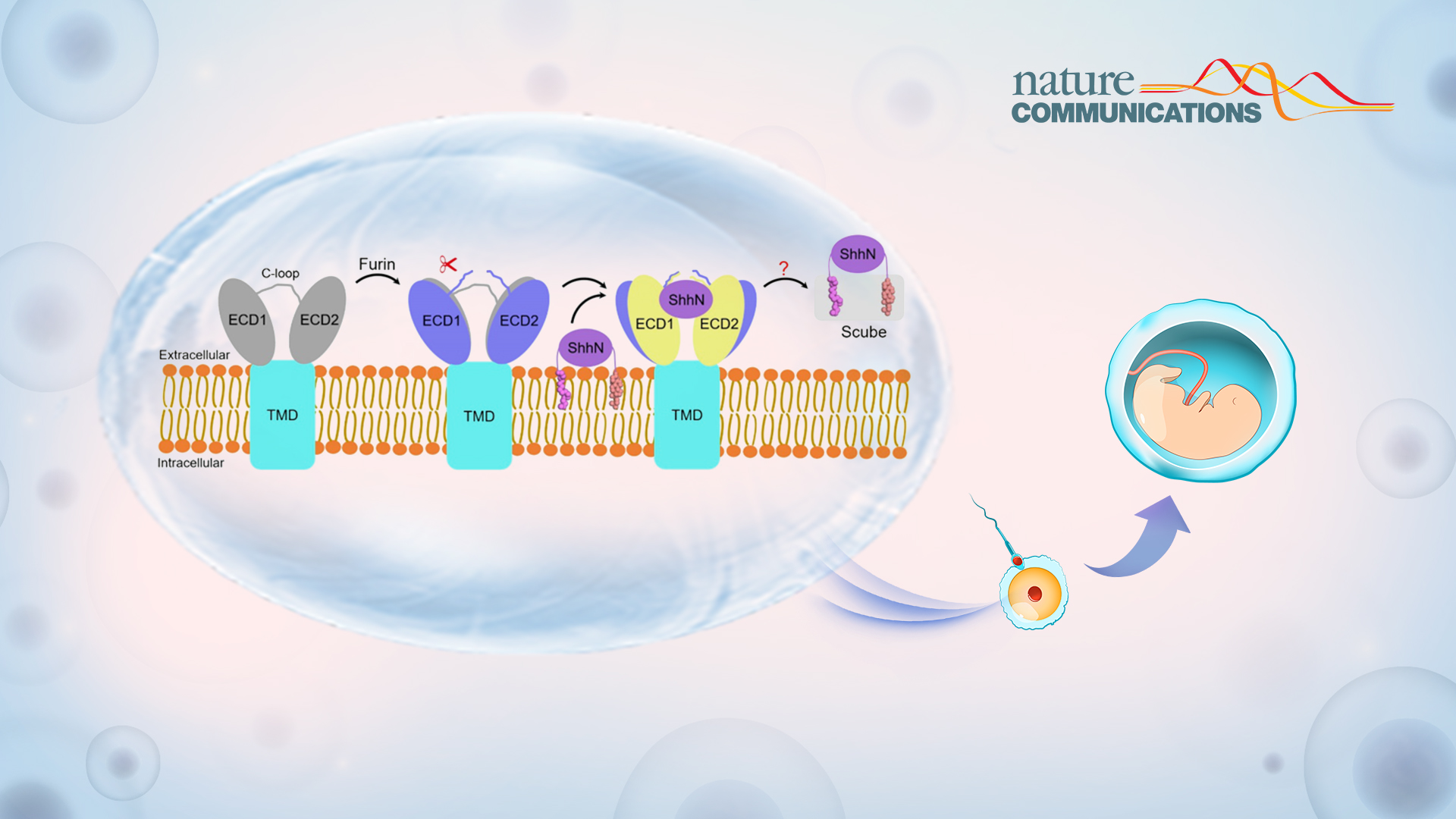
Release of lipid-modified Hh ligands, such as Sonic hedgehog (Shh), relies on a dedicated transport system involving the transmembrane protein Dispatched (Disp) and a member of the Scube family. Disp catalyzes the transfer of Shh from the membrane to the Scube acceptor through a postulated hand-off mechanism.
Subsequently, the secreted Scube-Shh complex diffuses extracellularly and delivers Shh to the surface of target cells. At this point, it ultimately binds its membrane receptor, Patched (Ptch), initiating signal transduction. The mechanism by which this proteolytic event leads to Disp activation still remains unknown.
Associate Professor Xin Gong’s group from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) recently published an article where they developed a strategy to obtain pure uncleaved (pre-proteolytic cleavage states) and cleaved (post-proteolytic cleavage states) human Disp1 (hDisp1) preparations.
Their study, entitled “Structural insights into proteolytic activation of the human Dispatched1 transporter,” was published in Nature Communications, a high-quality research journal in areas such as biological, health, physical, chemical, and Earth science.
Acquiring hDisp1 in pre-and post-proteolytic cleavage states
The researchers replaced the region in hDisp1 recognized by Furin (residues 263-280) with a cleavage site for the highly specific 3C protease (Fig. 1a-d). Cell-based Shh release assays combine affinity pull-down assay showing cleavage is important for hDisp1 activity, at least partly by controlling Shh binding.
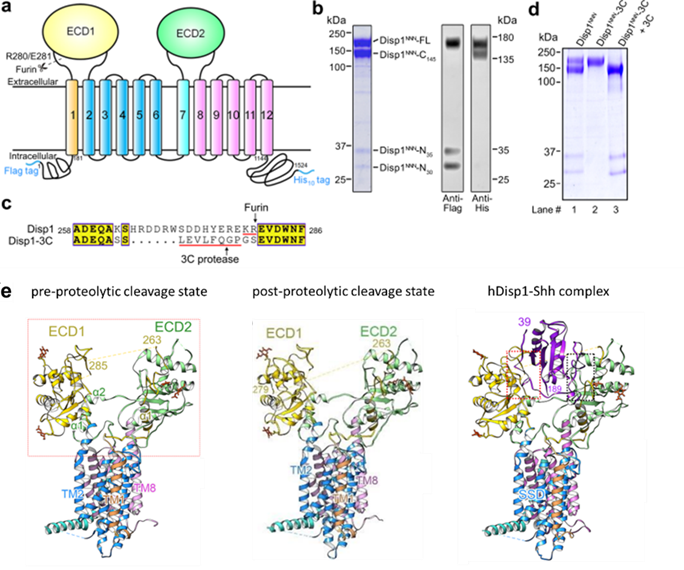
Figure 1. Purification and structural determination of human Disp1 in three different states
Next, the researchers obtained the cryo-EM structures of hDisp1 in pre-and post-proteolytic cleavage states, at overall resolutions of 3.61 Å and 3.68 Å, respectively (Fig. 1e, left and middle); and the cryo-EM structure of hDisp1 in complex with the native, dually lipidated Shh ligand, at an overall resolution of 4.07 Å (Fig. 1e, right).
A comparison between the structures of hDisp1 in pre-and post-proteolytic cleavage states reveals conformational shifts of the two ECDs, whereas the TMDs remain largely unchanged. After proteolytic cleavage, the two ECDs turn outwardly, away from each other, by approximately 2-3 Å, leading to a more open conformation of the extracellular surface of hDisp1. Cleavage also removes a steric block posed by an unstructured loop, allowing cleaved hDisp1 to bind Shh with significantly increased affinity.
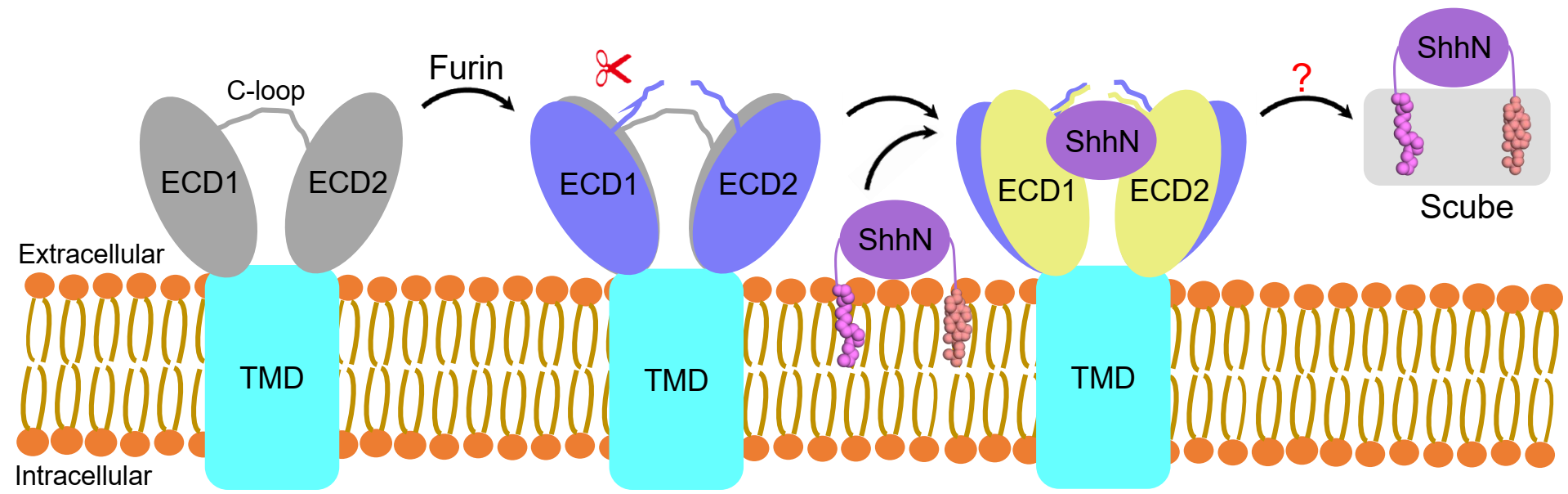
Figure 2. Model for human Disp1 activation by proteolytic cleavage
Wanqiu Li, Assistant Professor from the Department of Pharmacology at SUSTech, Linlin Wang, a Ph.D. candidate from the School of Life Sciences at SUSTech, and Dr. Bradley M. Wierbowski from Harvard University are the co-first authors of this paper. Prof. Xin Gong from SUSTech and Prof. Adrian Salic from Harvard University are the co-corresponding authors.
This work was supported by the National Natural Science Foundation of China (NSFC), the Natural Science Foundation of Guangdong Province for Distinguished Young Scientists, and the Shenzhen Science and Technology Plan. The Cryo-EM Center at SUSTech supported the Cryo-EM data collection and image processing.
Paper link: https://www.nature.com/articles/s41467-021-27257-w
latest news
-
Dynamic changes in transposable elements shape human three-germ-layer differentiation
Date:2025-09-04
-
Researchers collaborate to uncover how SOD1 protects lysosome through autophagy
Date:2025-08-26
-
Researchers find 5-IP7 disrupts intestinal epithelial barrier and drives inflammation-induced colorectal cancer
Date:2025-08-26
-
Researchers decode molecular architecture and inhibition mechanism of human taurine transporter
Date:2025-08-22
