RNA modification regulates nervous system development and functions
DATE:2021-10-25
N6-Methyladenosine (m6A) is the most abundant internal modification in mRNA. Previous research has shown that m6A is a reversible and dynamic RNA modification that is fundamental to the regulation of RNA metabolism. Therefore, m6A modification is a prevalent modification on mRNA, influencing splicing, export, translation, and decay.
A team led by Professor Shengjian Ji from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has conducted advanced research to explore the roles and mechanisms of m6A modification in nervous system development and functions. Their study reports a mechanism in which the m6A reader proteins regulate axonal mRNA local translation and further mediate axon growth in cerebellar granule cells (GCs).
Previous studies undertaken by the group showed that m6A modification plays an essential role in the early development of the nervous system. The eraser of m6A, FTO, regulates axon growth by controlling the local translation of GAP43 mRNA in axons (Yu et al., Nucleic Acids Research, 2018). Additionally, they also found that the reader protein of m6A, YTHDF1, mediates axon guidance by regulating the translation of the Robo3.1 mRNA (Zhuang et al., Nucleic Acids Research, 2019). However, how the m6A reader proteins regulate local translation of axonal mRNA remains unclear.
In their recent findings, the m6A reader proteins, YTHDF1 and YTHDF2, were found to be highly expressed in the axons of mouse cerebellar GCs. After the knockdown of YTHDF1 or YTHDF2, the axon growth rates of GCs were significantly increased. Using multi-omics analysis, Dvl1 and Wnt5a mRNA were identified as the key targets of YTHDF1 and YTHDF2, respectively.
Interestingly, Dvl1 and Wnt5a are the key components of the Wnt/Planar cell polarity (PCP) signaling pathway. They further discovered that YTHDF1 regulates the local translation of Dvl1 mRNA by promoting its translation, while YTHDF2 regulates the local translation of Wnt5a mRNA by affecting its stability. Thus, YTHDF1 and YTHDF2 synergistically control GC axon growth.
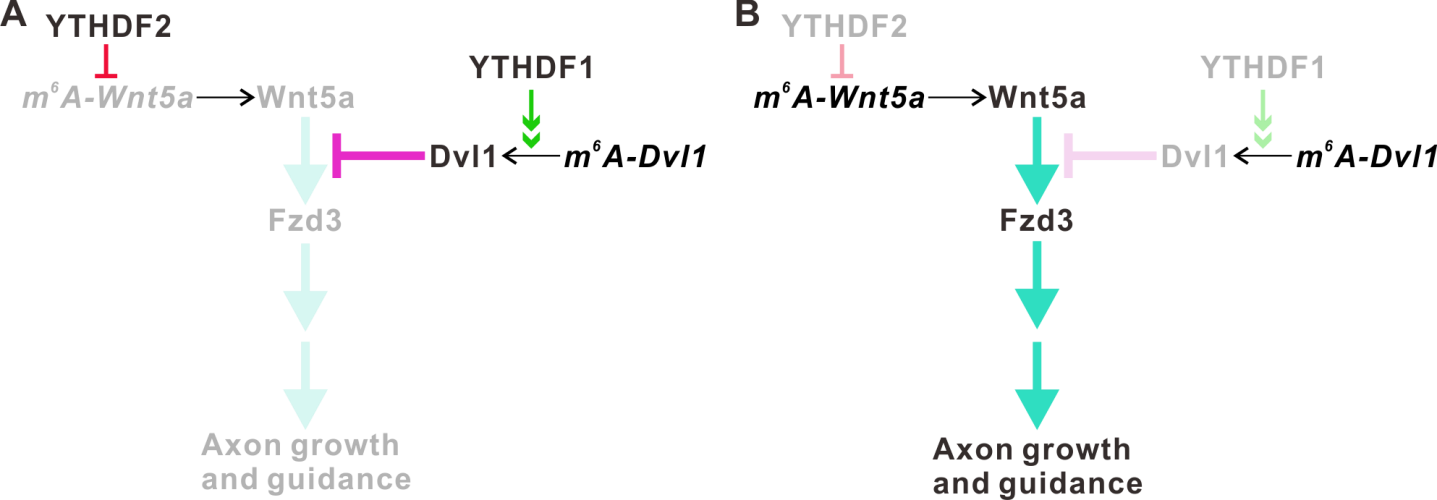
Figure 1. YTHDF1 and YTHDF2 work synergistically to regulate the Wnt5a-PCP signaling pathway and cerebellar granule cell axon growth
The team also tested this mechanism physiologically. Conditional knockout (cKO) of Ythdf1 or Ythdf2 in mouse cerebellar GCs significantly promoted cerebellar parallel fiber growth. Surprisingly, the number of synapses between parallel fiber and Purkinje cells was increased in either Ythdf1 cKO or Ythdf1 cKO mice. Subsequently, this caused the enhanced motor coordination ability in either cKO mice. These results showed that YTHDF1 and YTHDF2 serve as negative regulators for PF growth of cerebellar GCs under physiological conditions.
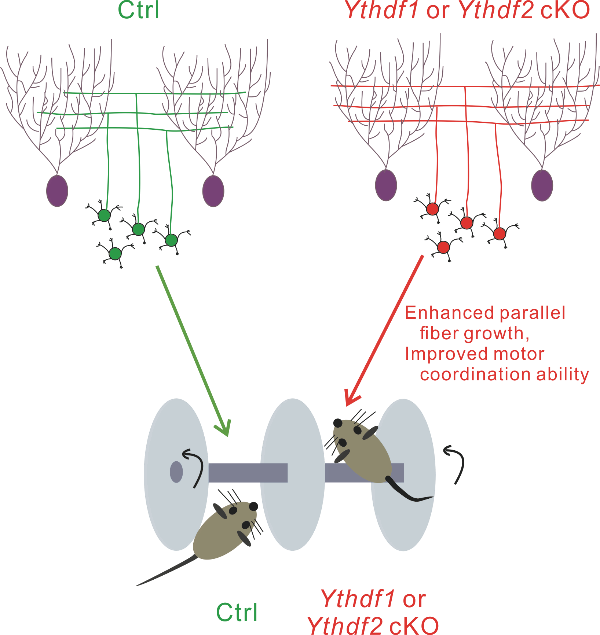
Figure 2. The parallel fiber growth and motor coordination ability are enhanced in Ythdf1 or Ythdf2 cKO mice
“Previous studies suggest that different m6A readers work reversely or redundantly. Here we show that the m6A readers YTHDF1 and YTHDF2 can work synergistically to regulate the same signaling pathway,” said Prof. Ji.
Their recent work is entitled “The m6A Readers YTHDF1 and YTHDF2 Synergistically Control Cerebellar Parallel Fiber Growth by Regulating Local Translation of the Key Wnt5a Signaling Components in Axons.” It has been published in Advanced Science, a renowned journal covering fundamental and applied research in materials science, physics and chemistry, medical and life sciences, and engineering. Jun Yu, a student in the joint Ph.D. program between SUSTech and the University of Hong Kong (HKU), Yuanchu She, a Ph.D. student at SUSTech, and Dr. Lixin Yang are the co-first authors of this paper. Prof. Shengjian Ji is the corresponding author.
Prof. Shengjian Ji’s group has made a series of significant findings that contribute to the field of m6A modification in neuronal development and functions. Consequently, they were invited to write a review paper on this topic by Frontiers in Cell and Developmental Biology, a broad-scope, interdisciplinary open-access journal, focusing on the fundamental processes of life. Jun Yu is the first author and Yuanchu She is the second author of this review paper. Prof. Shengjian Ji is the corresponding author.
The group also made a series of important findings that contribute to the field of local translation in axons. They recently published a review paper on this topic in Cellular and Molecular Life Sciences, a multidisciplinary journal covering the latest aspects of biological and biomedical research. Lichao Li is the first author, and Jun Yu is the co-first author of this review paper. Prof. Shengjian Ji is the corresponding author.
Jun Yu has published five research and review papers as the first/co-first author in internationally recognized journals, including Nucleic Acids Research, Development, Advanced Science, and Frontiers in Cell and Developmental Biology, and Cellular and Molecular Life Sciences. She was also awarded as the “Top 10 Graduates of 2021” at SUSTech. She is an exemplary example of the tremendous success of the joint Ph.D. programs set up by SUSTech and other top universities overseas.
Paper links:
Advanced Science:
Frontiers in Cell and Developmental Biology:
Cellular and Molecular Life Sciences:
latest news
-
Researchers develop high-yield plant chassis for green biosynthesis of soybean phytoalexins
Date:2025-05-29
-
TranslationAI: Deep learning model unveils hidden rules of RNA translation
Date:2025-05-07
-
Researchers collaborate to unveil leaf senescence and nutrient source-sink allocation at single-cell level
Date:2025-04-17
-
Scientists elucidate critical role of ATG2A in neural autophagy through regulating autophagosome-lysosome fusion
Date:2025-04-17
