Press Release Lactate: A “Double-Edged Sword” to Neurons
DATE:2021-10-08
The nervous tissues control every function we perform. Therefore, maintaining their structural and functional stability is essential to human health. And understanding the cellular and molecular mechanisms underlying the stability of the nervous systems has long fascinated neuroscientists. Previous studies indicate that neuroglia metabolically support neuronal function through the release of the glycolytic product--lactate and disruption of the metabolic coupling is implicated in neurodegeneration. The outstanding question is how glial lactate metabolism is regulated and dysregulated lactate metabolism is linked to neuronal metabolism and function.
Using nerve tissues of the peripheral nervous system (PNS), the research team led by Dr. Xiao, a professor at SUSTech School of Life Sciences, has tackled this question and found that a small GTP-binding protein known as Rheb plays a role in the regulation of glial lactate production through pyruvate. Research shows that Rheb-regulated mitochondrial pyruvate metabolism of Schwann cells (glia in the PNS) is critically linked to the local lactate homeostasis and persistently increased lactate input to neuronal axons leads to axon degeneration in the PNS. On October 6, this finding is published in the prestigious journal Developmental Cell.
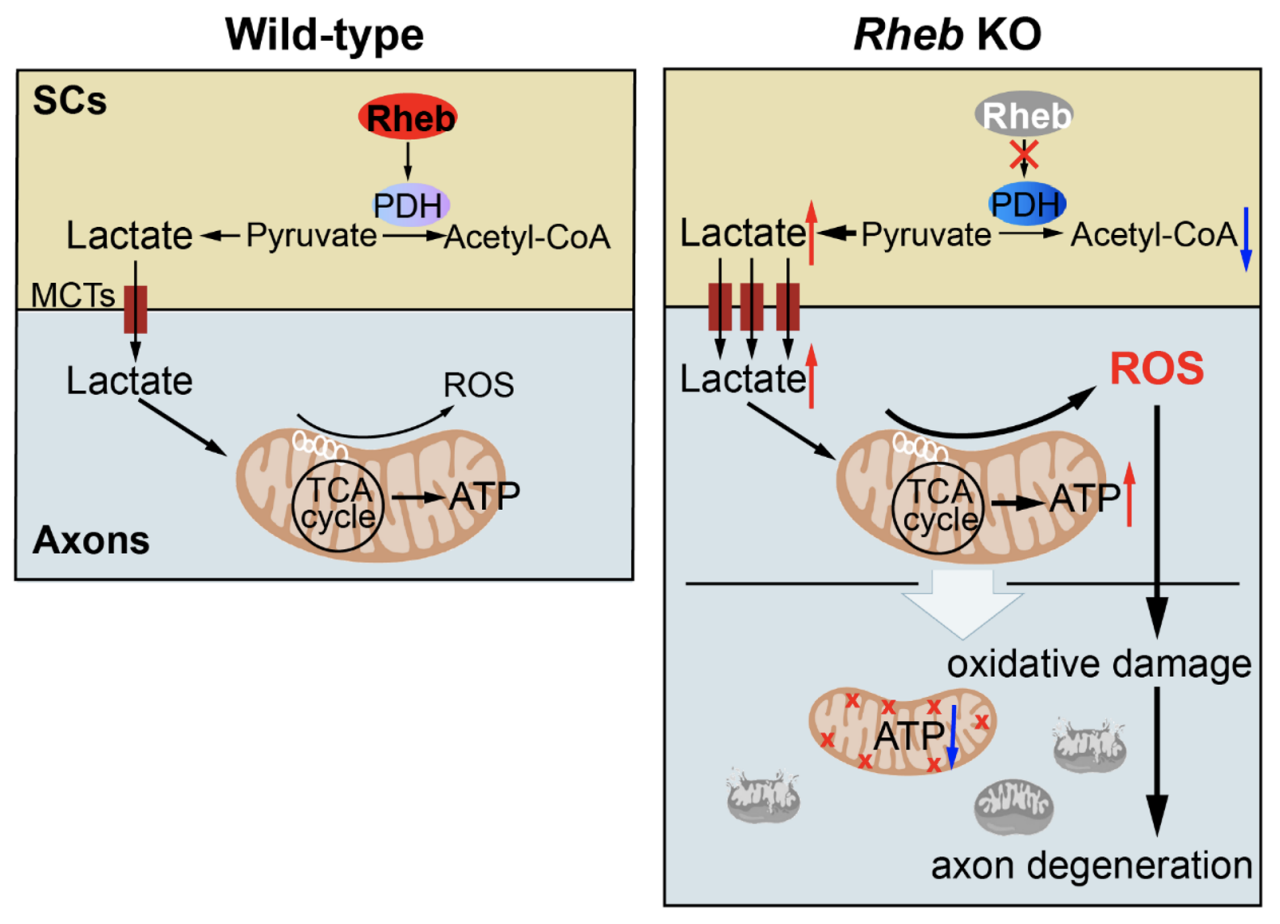
Building on their previous discovery of a role of Rheb in regulating mitochondrial metabolism (also reported in Developmental Cell, 2011), the research team genetically deleted Rheb in Schwann cells and found that genetic inactivation of Rheb gene in Schwann cells (SCs) hampers the conversion of pyruvate to acetyl-CoA by pyruvate dehydrogenase (PDH), and instead increases the conversion of pyruvate to lactate. The lactate Schwann cells export to neurons is rapidly metabolized to pyruvate perhaps in both the cytosol and the mitochondria. Increased lactate input to neurons enhances mitochondrial metabolic activity, producing more reactive oxygen species (ROS) and leading to neuronal oxidative stress. Oxidative stress damages mitochondria and impairs ATP production and further increases ROS production. This vicious cycle eventually leads to axon degeneration of the peripheral nerves. To prove that increased lactate input to neurons is a major culprit of the neuronal injury, they found that reducing the production or transport of lactate significantly ameliorates oxidative damage to the axons.
Also, the group found that loss of Rheb function in Schwann cell precursors has only a modest effect on the genesis of mature Schwann cells and their survival and peripheral myelination, thus enhancing the point that the neuronal damage is a direct result of disrupted local lactate homeostasis.
Lactate as an energy substrate is previously known to support neuronal function, preserve injured axons, and even promote axon regeneration. This study shows that lactate could be a “double-edged” sword; persistent influx of lactate at a higher rate to neurons is detrimental to neuronal function in the long haul. Increased lactate accumulation is commonly seen in disease conditions, e.g. diabetes and aging brain. The findings in this report could shed light into the pathogenesis of aging and neurodegeneration.
Prof. XIAO Bo and his student JIA Lanlan (the first author, a graduate student of State Key Laboratory of Biotherapy, Sichuan University, also a visiting student at SUSTech) conceived of and designed this study. JIA performed the bulk of the experiments. This study is funded by National Natural Science Foundation of China, Shenzhen Key Laboratory for Gene Regulation and Systems Biology, and Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institution.
Provided by ZHU Feiyan
latest news
-
Dynamic changes in transposable elements shape human three-germ-layer differentiation
Date:2025-09-04
-
Researchers collaborate to uncover how SOD1 protects lysosome through autophagy
Date:2025-08-26
-
Researchers find 5-IP7 disrupts intestinal epithelial barrier and drives inflammation-induced colorectal cancer
Date:2025-08-26
-
Researchers decode molecular architecture and inhibition mechanism of human taurine transporter
Date:2025-08-22
