SUSTech Bo Xiao’s research team discovers novel regulation of mitochondrial metabolism with implications to neuroenergetics
DATE:2021-03-30
Brains consume disproportionately a large amount of energy relative to their size. How brain cells handle energetic needs has fascinated scientists for ages. Research shows brain neurons are big energy spenders. Energy deficiency of neurons has serious consequences, for example, neurodegeneration. How neurons match energy consumption and production remains a puzzle.
A research team led by Professor Bo Xiao of the Department of Biology at the Southern University of Science and Technology (SUSTech) has discovered a new mechanism by which neurons (and other types of cells) regulate mitochondrial activity and couple neural activity to increased ATP production to meet neurons’ energetic needs. This new mechanism puts the small GTPase Rheb on the frontline in activity-regulated neuroenergetics. This research article has been published in the March 22 edition of the prestigious journal Developmental Cell, entitled “Rheb Mediates Neuronal-Activity-Induced Mitochondrial Energetics through mTORC1-Independent PDH Activation”.
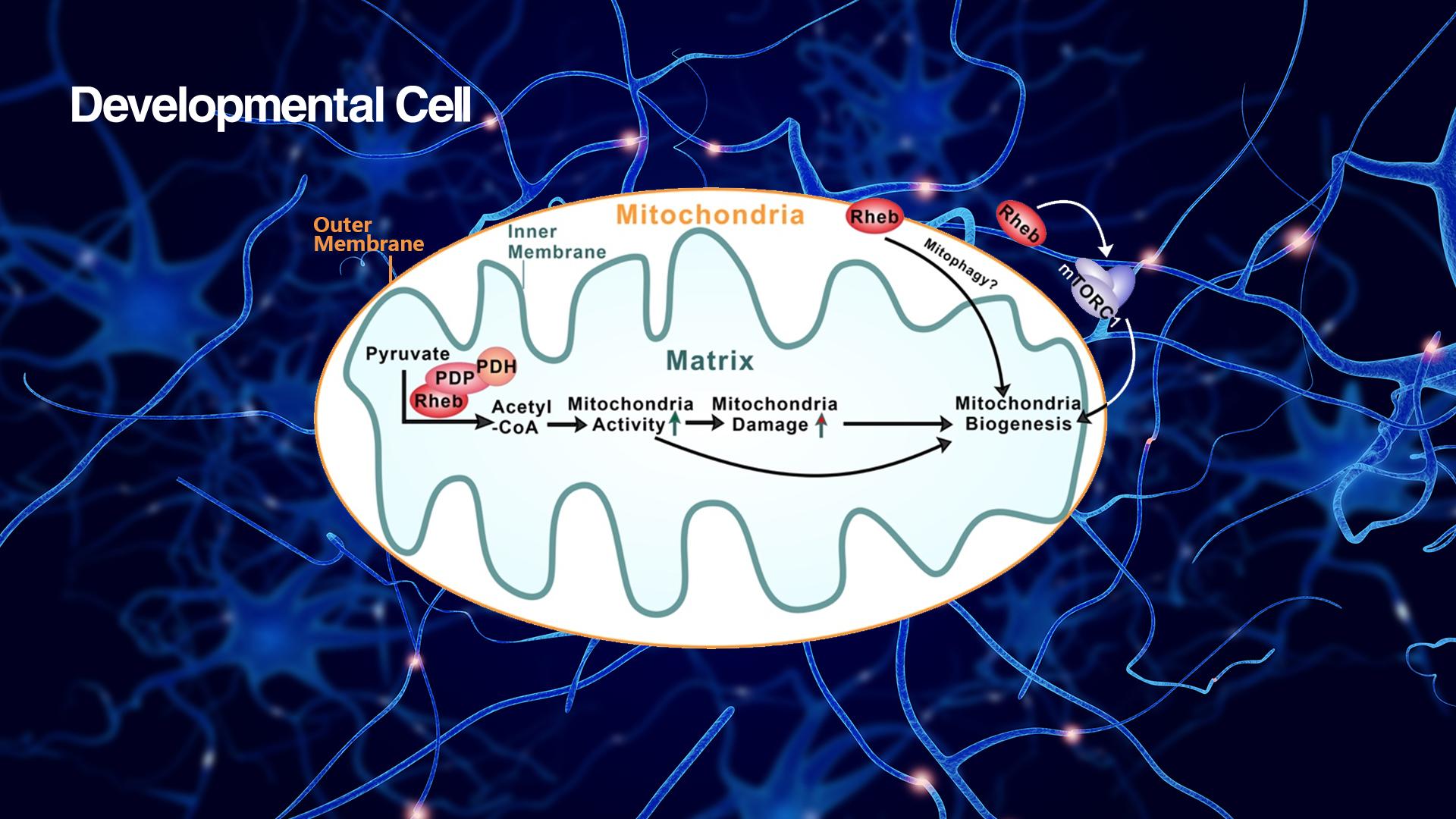
The team reports that Rheb plays a role in controlling the activity of the enzyme complex known as pyruvate dehydrogenase (PDH) that catalyzes pyruvate to acetyl-CoA conversion. Genetic deletion of the Rheb gene in cells reduces acetyl-CoA and ATP production. This role of Rheb does not involve its well-known role in activating mTORC1, because inhibiting mTORC1 by applying the commonly used mTORC1 inhibitors rapamycin and torin1 to cells did not alter the phosphorylation status and activity of PDH. Additionally, selective deletion of mTOR and raptor (a component of mTORC1), respectively in cells, does not affect PDH activity indicated by its phosphorylation status. Instead, this novel function of Rheb involves its binding to PDH phosphatase (PDP) and the stabilization of the Rheb-PDP-PDH complex (Figure 1).
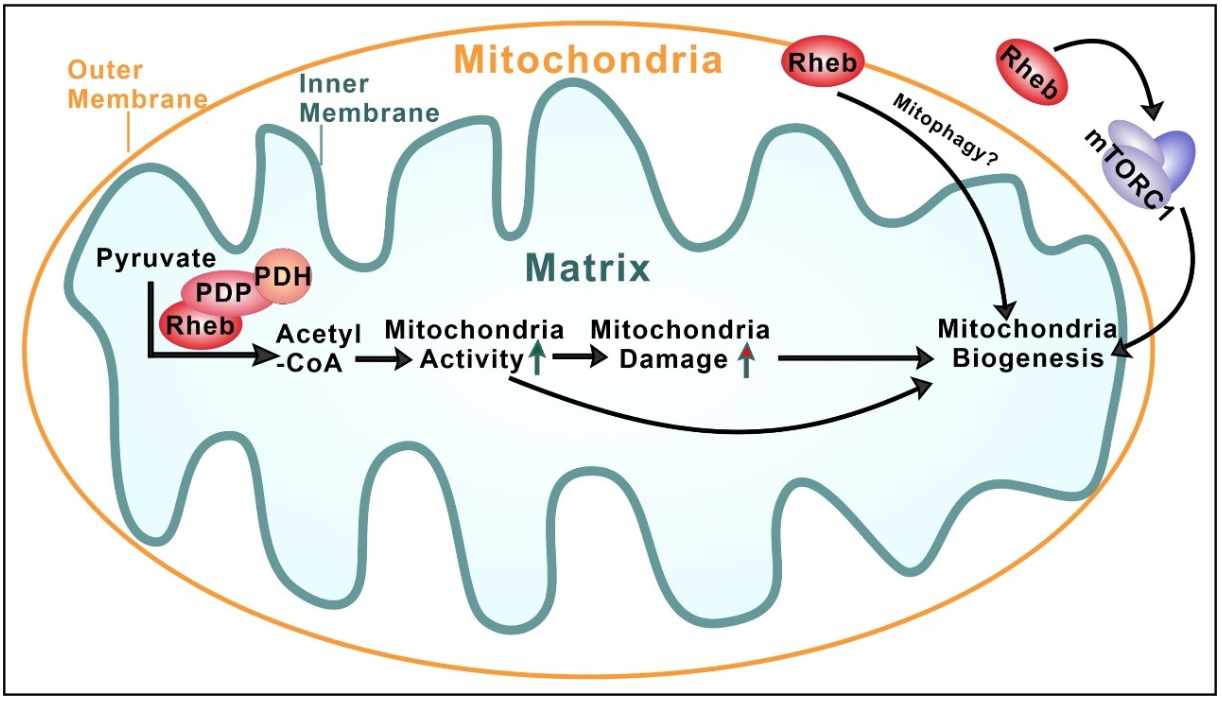
Figure 1. Rheb binding to PDH phosphatase (PDP) and the stabilization of the Rheb-PDP-PDH complex
The Rheb-PDP-PDH axis suggests Rheb’s presence in mitochondria, specifically in the mitochondrial matrix. To demonstrate Rheb’s localization in the mitochondrial matrix, the researchers imaged Rheb binding to PDP in the mitochondrial matrix, using proximity ligation assay (PLA), besides biochemical fractionation of purified mitochondria. Furthermore, they showed that Rheb dynamically traffics to mitochondria via its interaction with Tom20, a translocase on the mitochondrial outer membrane.
Earlier work pioneered by Professor Paul Worley of Johns Hopkins University, a co-corresponding author of this study, shows the neuronal expression of Rheb is induced by neural activity. Building on the inducibility of Rheb by neuronal activity, this study further investigated the correlation of activity/metabolic input and Rheb expression and PDH activity and if neuronal activity induced neuronal ATP production is dependent on Rheb. Findings indicate that Rheb, together with PDH activity, is induced by activity and metabolic needs, and plays a role in activity-induced neuronal energetics (Figure 2).
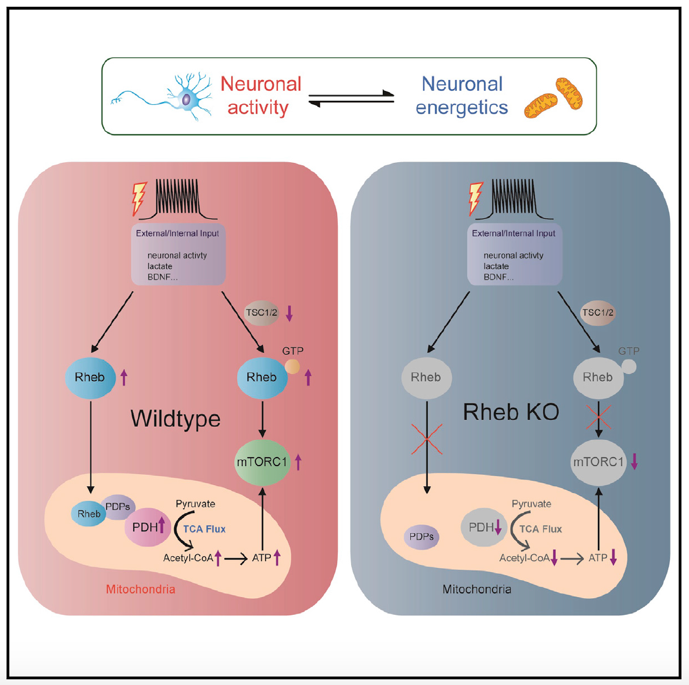
Figure 2. Rheb, together with PDH activity, induced by activity and metabolic needs
The Rheb-regulated PDH appears to be a general mechanism cell that adapts to regulate energy production. Given the essential roles of acetyl-CoA in multiple cellular processes of metabolism and gene regulation, this exciting discovery of Rheb-regulated acetyl-CoA production would have a broad impact on multiple areas of biomedical research.
Professor Bo Xiao of the Department of Biology at SUSTech is the first author of this paper. Professor Paul Worley of Johns Hopkins University is the co-corresponding author of this paper. This work was funded by the National Natural Science Foundation of China (NSFC), Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions, and Shenzhen Innovation Committee of Science and Technology Grants.
Paper link: https://doi.org/10.1016/j.devcel.2021.02.022
latest news
-
Dynamic changes in transposable elements shape human three-germ-layer differentiation
Date:2025-09-04
-
Researchers collaborate to uncover how SOD1 protects lysosome through autophagy
Date:2025-08-26
-
Researchers find 5-IP7 disrupts intestinal epithelial barrier and drives inflammation-induced colorectal cancer
Date:2025-08-26
-
Researchers decode molecular architecture and inhibition mechanism of human taurine transporter
Date:2025-08-22
