SUSTech researchers reveal molecular mechanisms to understand how cells communicate with surrounding environments
DATE:2021-04-19
In multicellular organisms, each cell needs to communicate with surrounding cells and the environment to fulfill its functions and these communications are mainly mediated by cell adhesions. Cell adhesions are critical for the migration of cancer cells and the formation of neuronal networks in the brain. However, the molecular mechanisms underlying the formation and regulation of cell adhesion remain elusive.

Recently, Associate Professors Cong Yu and Zhiyi Wei from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) published two research papers in Cell Reports on March 23, 2021, entitled “Oligomerized liprin-α promotes phase separation of ELKS for compartmentalization of presynaptic active zone proteins” and eLife on February 15, 2021, entitled “Complex structures of Rsu1 and PINCH1 reveal a regulatory mechanism of the ILK/PINCH/Parvin complex for F-actin dynamics” to unveil the molecular mechanisms underlying two types of cell adhesions.
Integrin-mediated focal adhesions (FAs) are formed by the cell and extracellular matrix, which mediate bidirectional signal transduction inside and outside the cell. The research team provides structural and cellular evidence to show how the FA proteins control the cytoskeleton organization at the FAs and regulate the FA dynamics via protein-protein interactions. On the other hand, synapses are another important type of cell adhesion that is formed between neurons and their target cells for neuronal signal transmission. Using structural biology and biochemistry methods, the team revealed that the core synaptic proteins, such as liprin-α and ELKS, can form protein condensates through liquid-liquid phase separation and control the protein distributions in the highly specialized synaptic structure.
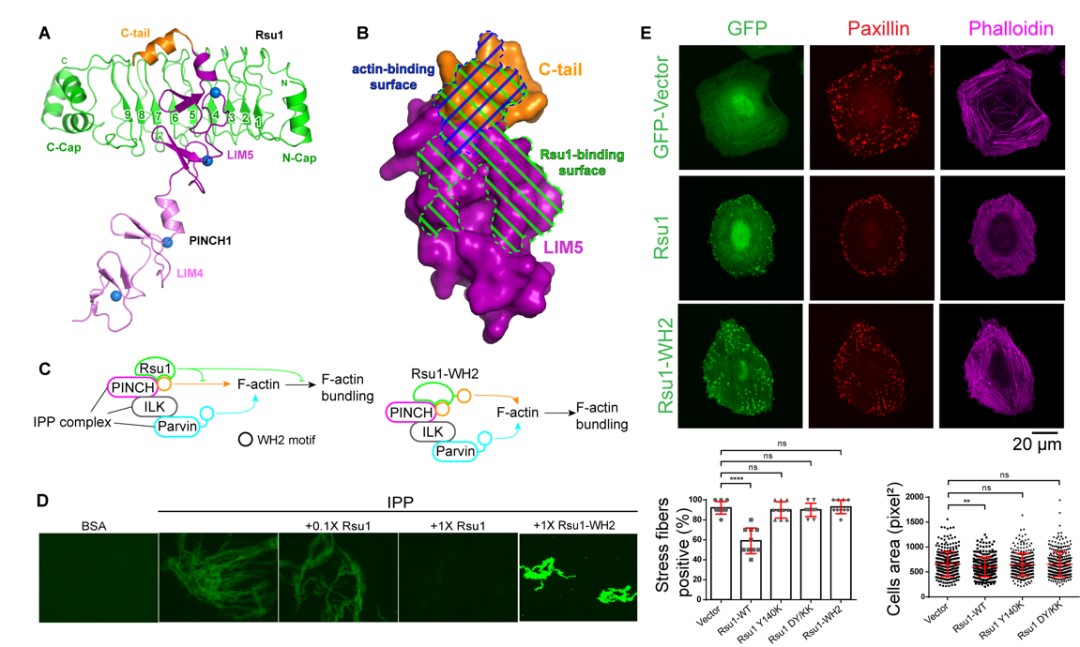
Figure 1. (A) Three-dimensional structure of PINCH1 and Rsu1 complex. (B) The binding site of Rsu1 and PINCH1 blocks the binding site of actin on PINCH1. (C) The schematic diagram shows that the combination of Rsu1 and PINCH1 inhibits the function of IPP-mediated F-actin bundles, and the designed chimeric mutant of Rsu1-WH2 can relieve this inhibitory function. (D) In vitro biochemical experiments verify the effects of Rsu1 and Rsu1-WH2 on F-actin bundles. (E) Cell data show the effect of Rsu1 on cytoskeleton regulation and cell movement.

Figure 2. Schematic diagram of Liprin-α mediated modular and regional assembly of multiple active zone proteins in the presynapse
Their works lays the foundation for understanding the occurrence of cancers and the neuronal connectivity.
Ph.D. student Haibin Yang and Leishu Lin from Dr. Yu and Dr. Wei’s laboratory respectively are the co-first authors of the eLife paper. Research Assistant Mingfu Liang from Dr. Wei’s laboratory, Ph.D. student Gaowei Jin from Dr. Yu’s laboratory, and Research Assistant Professor Xingqiao Xie from the Academy for Advanced Interdisciplinary Studies at SUSTech are the co-first authors of the Cell reports paper. Dr. Yu and Dr. Wei are the corresponding authors of these two papers.
This work was supported by the SUSTech Core Research Facilities and BL17U, BL18U, and BL19U1 beamlines of the Shanghai Synchrotron Radiation Facility (SSRF) for data collection. This work was funded by the National Natural Science Foundation of China (NSFC), Science and Technology Planning Project of Guangdong Province, the Natural Science Foundation of Guangdong Province, Shenzhen-Hong Kong Institute of Brain Science, Shenzhen Fundamental Research Institutions, and Shenzhen Science and Technology Innovation Commission.
Paper links:
https://elifesciences.org/articles/64395
https://www.cell.com/cell-reports/fulltext/S2211-1247(21)00215-1
latest news
-
SUSTech Researchers Uncover the Molecular Mechanism of C1ql1/BAI3 Assemblies in CF-PC Synapse Development
Date:2025-12-29
-
Researchers discover mechanisms underlying how gravity competes with vision to guide brain’s inner compass
Date:2025-09-22
-
Dynamic changes in transposable elements shape human three-germ-layer differentiation
Date:2025-09-04
-
Researchers collaborate to uncover how SOD1 protects lysosome through autophagy
Date:2025-08-26
